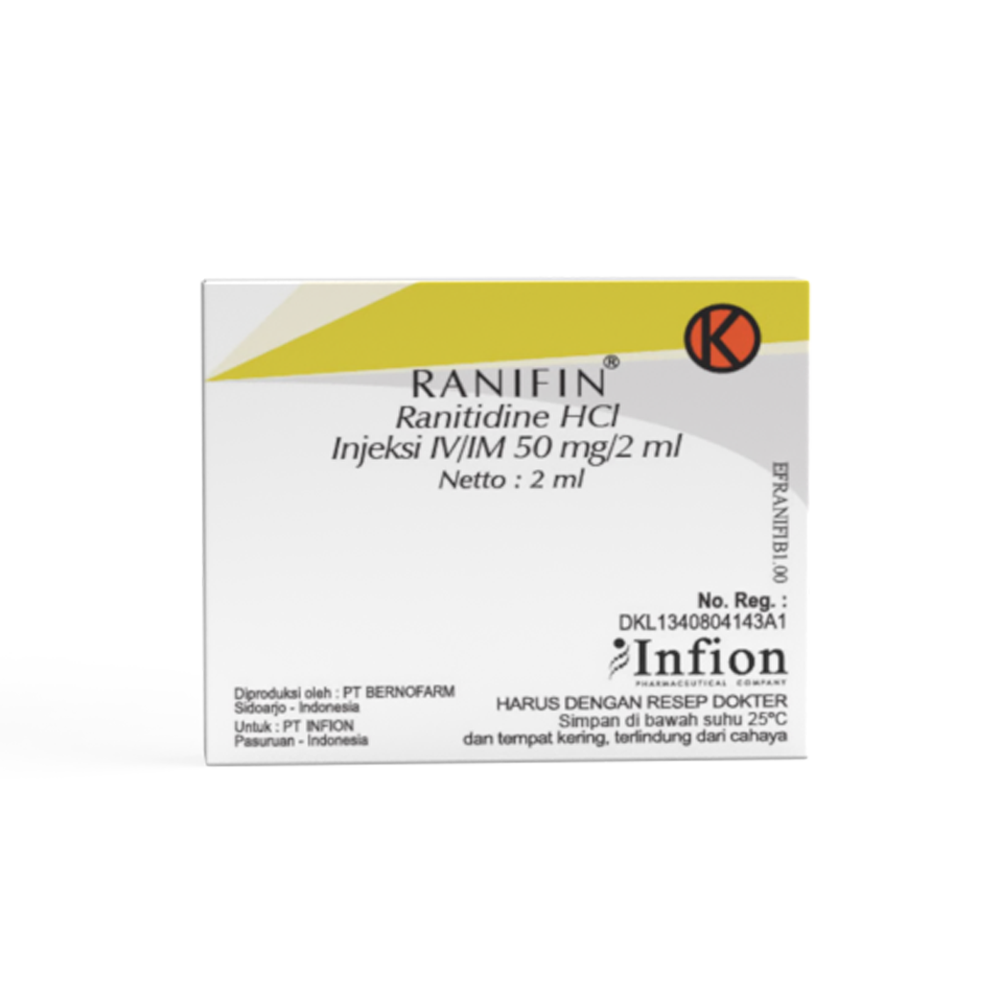
Ranifin®
Ranitidine
Indication : For hospitalized patients with pathological hypersecretory conditions or intractable duodenal ulcers, or as an alternative to the oral dosage form for short-term use in patients who are unable to take Ranitidine oral.
Composition : Each ml Ranifin® IV/IM Injection 50 mg/2 ml contains Ranitidine HCl equivalent to Ranitidine 25 mg.
Contain of excipients: Dibasic sodium phosphate dihydrate, Potassium phosphate monobasic, water for injection.
Presentation : Ranifin® IV/IM Injection 50 mg/2 ml
Box, 5 ampoules @ 2 ml
On medical prescription only.
Store below 25°C and dry place, protect from light
Other Related Products
Related products
-
Ganion®
Gastrointestinal & Hepatobiliary Systems -
Vomiper® Drops
Gastrointestinal & Hepatobiliary Systems -
Esofin®
Gastrointestinal & Hepatobiliary Systems -
Dansefion® Syrup
Gastrointestinal & Hepatobiliary Systems